

|
||||
| Home | Services | CDISC | Software | About us |
Pharmaceutical companies more and more submit information to regulatory authorities (such as FDA and EMEA) in electronic form. Especially EMEA encourages companies to sumbit the information in electronic form and especially in XML-format.
One of the apects of XML is that it is international: within one document, information in (many) different languages can be stored. This is especially interesting for submitting information in an international - multilangual environment, such as EMEA.
Typically, information to EMEA is submitted in about 11 languages
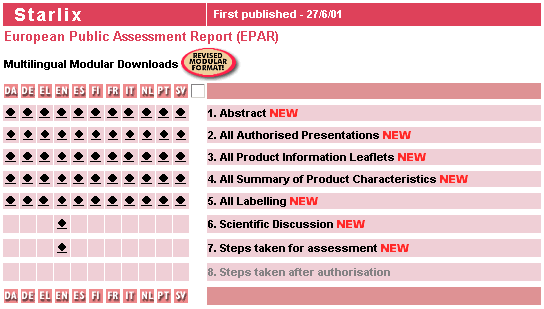
In the past, this was typically done by submitting 11 separate dossiers, with all problems of synchronization (the dossiers should contain exactly the same information). With XML, the data can be stored within one XML-dossier, with tools to controll the consistency and synchronization between different languages.
Typically, the time needed to construct a full dossier, is reduced by about 40-50%. This means faster commercialization, longer patent protection, so ... more profit.
