

|
||||
| Home | Services | CDISC | Software | About us |

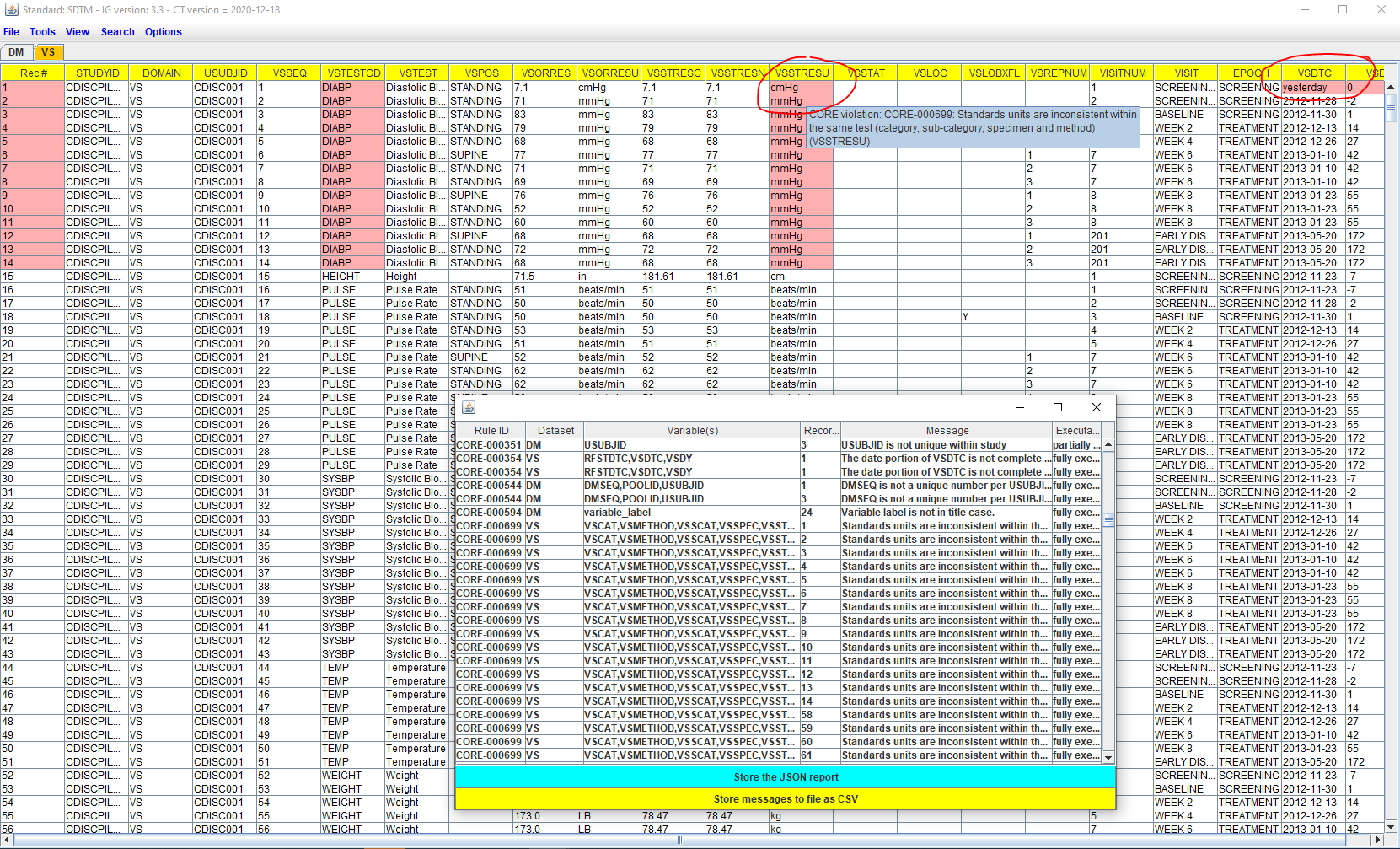
CORE (CDISC Open Rules Engine) is a project of CDISC to generate and deliver "real open rules" for SDTM, SEND and ADaM validation (and more), as well as an engine for it. "Real Open" means that the source code of the rules themselves (written in YAML or JSON) can be inspected, and that the execution engine (written in Python) can be downloaded, changed (when necessary), installed on any machine one wishes and used as one wishes, including implementation in existing software, and all this without any licensing limitations. This is the complete opposite of the validation software that is mostly used for submission datasets validation in our in industry, which is completely closed, with an intransparent implementation of the rules ("black box"), and notoriously known for its false positives and incorrect interpretation of the CDISC rules.
CORE is developed in close cooperation with the FDA, and is expected to soon replace the validation software currently used by the FDA. It has also already been introduced to the PMDA and to the NMPA.
Besides being completely "open", CORE has a number of advantages over other submission validation software:
For example, we generated "quality rules" starting from the "SDTM Dataset Specializations", which are essentially "SDTM implementations" of the "CDISC Biomedical Concepts".
We are also making these "SDTM Dataset Specialization custom" rules available to the industry.
Several examples can be found here.
XML4Pharma has been extremely actively involved in the development of CORE, especially in the implementation in YAML of hundreds of rules for SEND, FDA Business Rules and SDTM, and in the testing of many of the rules developed by other volunteers. As such we can say we are "expert rule developers".
We thus recently started providing CORE services to organizations and companies:
We find knowledge and technology transfer important. We are not one of these consultants or technology providers that provide you with a black-box solution.
At the contrary, during an assignment, we learn your people understanding the CDISC standards and how to work with them.
When developing a software solution specific for your company, we provide the source code of that software together with the full software documentation.
