

|
||||
| Home | Services | CDISC | Software | About us |
Other tools require you to provide details of the define.xml (after having generated a prototype from the XPT files) using an Excel worksheet. No manual how to do this is however provided. It is suggested to take an existing define.xml and to generate an Excel worksheet from that and edit the latter, adapting it for your own study, and then generate a new define.xml for your own study. A doom loop indeed! Other tools use an "Excel-like" GUI, but still have the "black box" issue. In the time you have learned how that works (and leading to a result that you do not understand yourself - as it is "black box"), you will already have done the job using the ODM Study and Define.xml Designer, AND obtain a define.xml that you do understand.
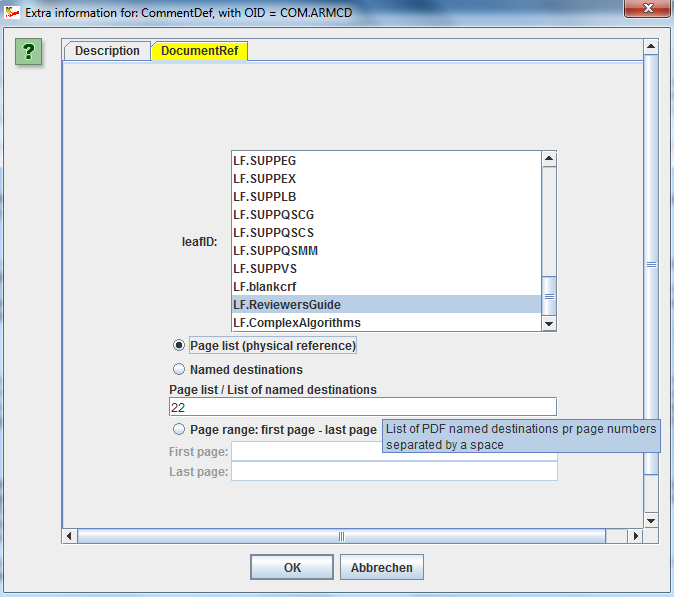
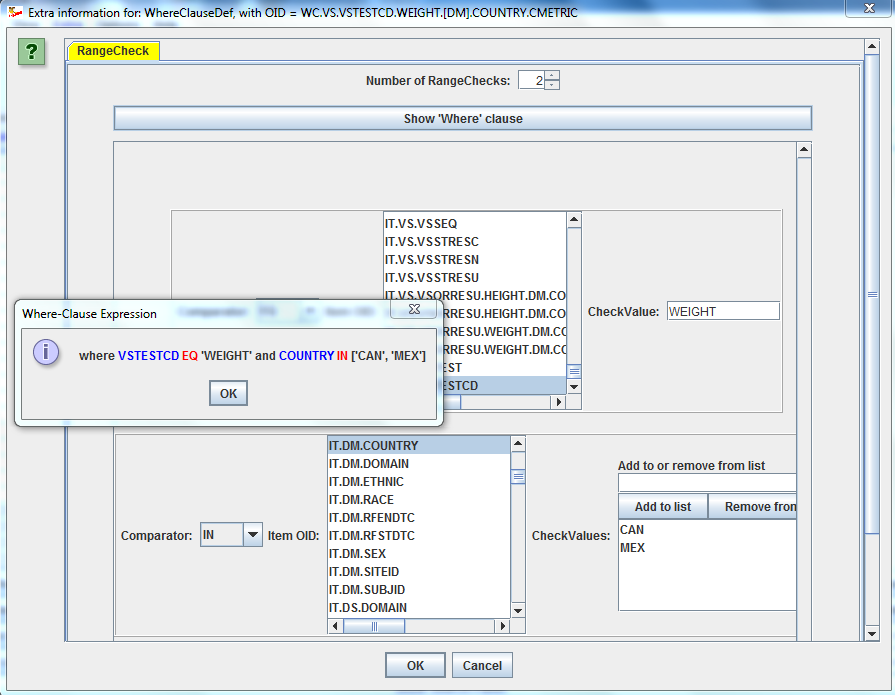
Using Excel (or an Excel-like GUI) for providing the details of the define.xml is extremely user un-friendly. The ODM Study and Define.xml Designer uses Wizards to allow you to specify details of the define.xml, for example to specify links to an external document for your SDTM/SEND/ADaM variable or comment (left picture) or to add all necessary "Where clauses" (right picture) in an extremely user-friendly way:
 |
 |
Want to know more? Then simply download the User Manual (regularly updated and extended). It contains very good explanations of all the features of the software.
When working in the define.xml mode, you will always be able to inspect what you have generated.
With just two clicks, you will be able to see the generated define.xml in your favority viewer, either using the CDISC stylesheet, or using your own stylesheet.
Even more, you will ALWAYS be able to inspect the underlying XML that is generated in the background (so NOT "black box"), or obtain a tree view of your study.
Unlike in other tools, validation of your define.xml is done using the rules developed by CDISC Define-XML team (and not the interpretation of the standard by a single vendor). For example, the software validates using the guidelines in the "XML Schema Validation for Define.xml White Paper", published by CDISC.
If your company does not implement "CDISC end-to-end" yet, and you only want to use the software to set up or generate define.xml files (either from scratch, SDTM/SEND templates or starting from a set of SAS-XPT files), we can offer you the "Define-XML" version of the software. This version does not allow you to do ODM study designs, but it has all the necessary features (which is already an enormous amount) for generating and working with define.xml (both v1.0 or v2.0).
Even more ideal than needing to design the define.xml "offline" using a tool like the ODM Study and Define.xml Designer, is to have a process in place that creates the mapping between operational data and the SDTM/SEND domains and datasets, and that automatically generates and synchronizes the define.xml in the background. Our SDTM-ETL software is the ideal tool for this as has already been discovered by many sponsors and service providers. Also SDTM-ETL is NOT a black-box tool as one always can inspect the underlying define.xml source. Just like the ODM Study and Define.xml Designer, it uses many Wizards to allow the users to specify the details of the define.xml in a very user-friendy way.
For more information, or if you would like to obtain a copy of the trial version, or obtain pricing information, do not hesitate to send us a mail.
