|
||||
| Home | Services | CDISC | Software | About us |

PIM (Product Information Management) is a project of the European Regulatory Authorities (EMEA) to have a standardized, electronic process of submitting and reviewing product information for new and existing pharmaceuticals.
PIM started in 1999, and after a long testing and development period, has now reached version DES 2.3.2 (July 2007). As listed on the EMEA website, the use of PIM in an electronic form has many advantages for pharmaceutical applicants:
Already in 2001, when still working at Organon as IT group leader with additional responsibility for IT-innovation,
Jozef Aerts (now at XML4Pharma) was the technical driving force behind the first large PIM submission in Europe.
Together with his colleague of the Regulatory Department, they started up a project team, educated the regulatory people in the use of XML,
selected and installed the necessary software, and did the PIM submission in considerable less time than usually necessary for such a submission.
Furthermore, Jozef initiated a software project for allowing local companies to perform the necessary translations online, using a web-based, intranet system.
Later (at XML4Pharma) Jozef specialized in helping pharmaceutical companies with the implementation of XML-based technologies. This means that he has experience in the use of XML for pharma R&D since XML was established as a W3C standard !
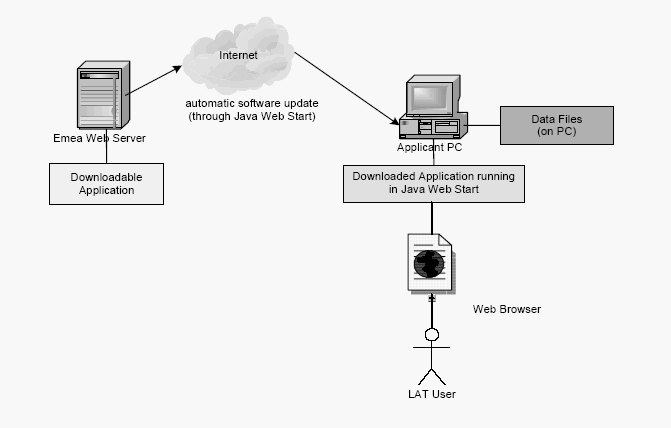
EMEA developed a web-based authoring tool for viewing, editing and life-cycle management of PIM XML-files: the Light Authoring Tool (LAT). The first release was in early 2006 (version 1.0 with basic functionality). The latest version (July 2007) is v.3.1.1. The tool is based on Java-technology, i.e. Java application server, JSP and Java Web Start.
XML4Pharma does currently not provide any PIM-related services anymore