
|
||||
| Home | Services | CDISC | Software | About us |
 |
Map your studies to SDTM or SEND |
The SDTM-ETLTM software is considered to be the lowest cost - highest benefit software for creating SDTM and SEND datasets in the industry.
All that is required is that your EDC system can export clinical data in CDISC ODM format (most EDC systems do so).
SDTM-ETL is completely "SAS®-free", i.e. unlike other solutions, you do not need an (expensive) SAS® license, nor do you need any statistical software.
SDTM-ETL comes with an extremely user-friendly graphical user interface, allowing to create most of the mappings by drag-and-drop or per mouseclick. At the same time, your define.xml (2.0 or 2.1) is generated automatically - details are to provided using intelligent wizards (no XML editing nor user-unfriendly Excel worksheets necessary).
Many CROs and service providers have already discovered SDTM-ETL and are using it for preparing their submissions to the regulatory authorities.
SDTM-ETL v.4.4 comes with a lot of new features and improvements, such as a implementation of the "CDISC-CT Relations", additional filtering on "looping" variables, and automated generation of VISITNUM for "unscheduled" visits
The most important documentation and a number of manuals is now available online:
Manuals and tutorials for new features introduced in v.4.4:
Sneak preview tutorials next version 4.5
Manuals developed on request of our customers:
Please notice that these manuals are constantly being improved and extended, so the contents can always change without special notice.
The problem
History
Overview
Features
Extract, Transform and Load
Advantages over other ETL software tools
Product information
Instruction movies
Training
Starting from Excel or CSV
Licensing
The problem
Setting up and executing a mapping between your clinical data and the CDISC SDTM Standard is not an easy task.
For some SDTM Variables, a 1:1 mapping is possible, but usually, clinical data items need to be combined to come to an SDTM Variable (n:1 mapping).
Furthermore, codelists need to be mapped, and clinical data categorized to SDTM codelists. Unfortunately, until now there was no software package on the market which could do this all at the same time in a user-friendly way. Therefore, XML4Pharma developed SDTM-ETLTM, a visual, easy to use software package for setting up mappings (and executing them) between clinical data in your operational database and the SDTM or SEND standard, at the same time generating the define.xml in the background. Define.xml (2.0 or 2.1) details can then be added using intelligent wizards (no user-unfriendly Excel files necessary). |
 |
 |
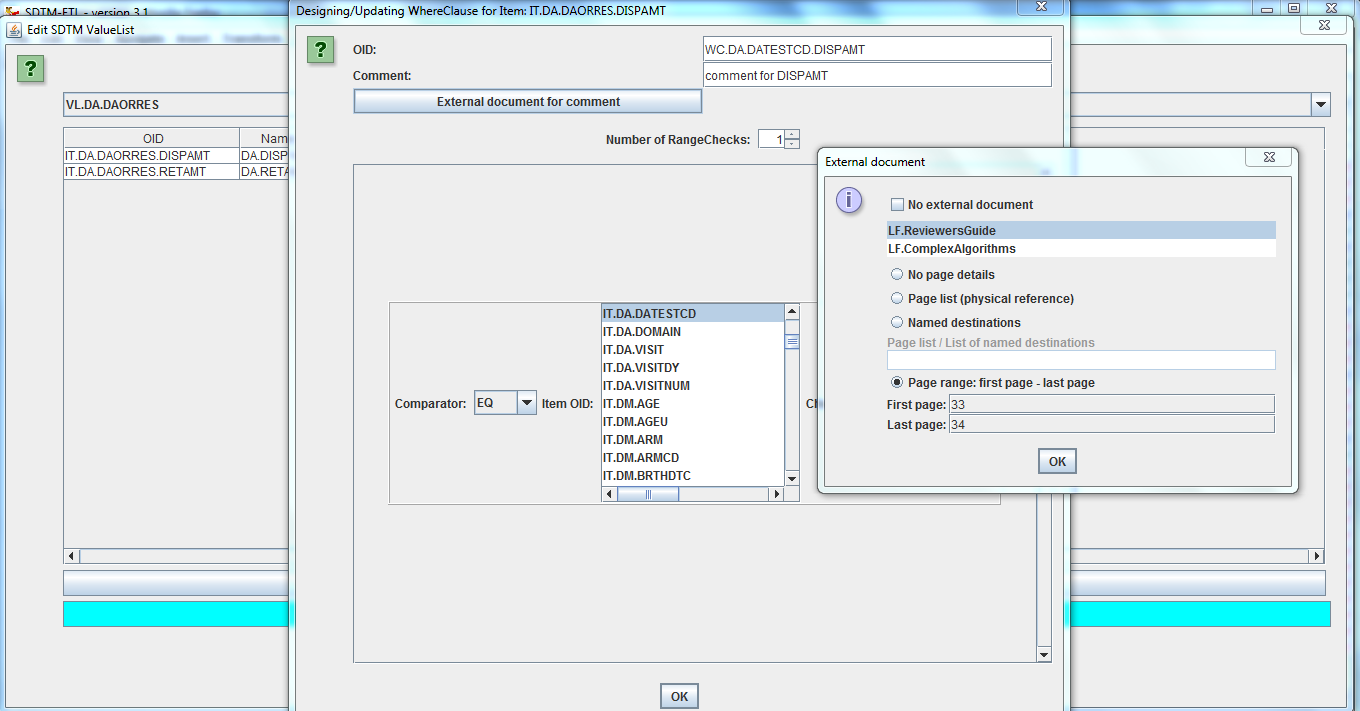
SDTM-ETLTM acts on CDISC ODM files (v.1.2, 1.3 or 1.3.1) which can easily be exported from any modern EDC, CDM system or database (the CDISC ODM standard is
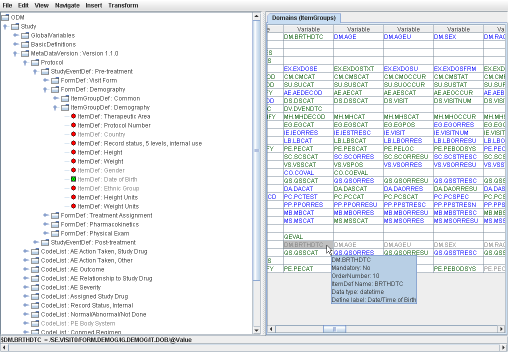
THE exchange format for clinical data between sponsors, CROs and the FDA). If your EDC system cannot export in ODM, or you receive your datasets in another format (Excel, CSV, SAS-XPT) have a look at our software to generate ODM from these. In SDTM-ETLTM, the clinical data metadata are represented as a tree on the left side of the screen, whereas the SDTM standard is represented as a table on the right side of the screen. Mappings can be created simply by drag-and-drop, wizards showing up automatically, making the mapping process very intuitive and easy. The mappings are then stored in an easy-to-learn scripting language within the underlying define.xml itself. This underlying define.xml is updated "on the fly", and kept in sync with all the additions and changes that are made during the mapping. |
SDTM-ETLTM is a real ETL tool - but specializing in SDTM. Once a mapping generated, clinical data can be extracted from ODM files, transformed into SDTM records, and loaded into the created SDTM database.
As such, SDTM-ETLTM, together with our Study Designer and our other product offerings, is one of the first real realizations of CDISC end-to-end.

More product information is available in the product flyer, and in the product brochure.
A set of instruction movies is available on our demo application server.
This set of instruction movies is regularly extended and improved. So we invite you to visit the 'instruction movies' website regularly.
Although the use of the SDTM-ETL software is very intuitive, many of our customers desire to get some training to start working with the software.
In our experience, one day of training usually suffices to make a jumpstart with the software. Such trainings are of course offered by us, usually at the location
of the customer.
A copy of the tutorial, explaining many of the features of the software, and used during trainings, is available on request.
We are currently also developing online training materials
The SDTM-ETL software is available at highly competitive prices. A perpetual license costs considerably
less than the SAS basic windows Analytics package first year license.
Considerably volume discounts can be obtained. Academic licenses are available at a reduced price.
For more information about licensing options and pricing, please contact us.
The SDTM-ETL software uses CDISC ODM as the source as this is the worldwide standard for exchange and archival of clinical data:
all modern EDC systems allow to export CDISC ODM metadata and clinical data.
In the seldom case that your data capture system does not export to ODM, one can always start from CSV (comma-separated-value) or SAS Transport 5 (XPT) data and transform these to ODM using our "ODM Generator" software.
The latter allows to generate ODM from any "text based" data using any kind of delimiter between the fields, or starting from SAS-XPT files.
This is also the best tool when your clinical study data is kept in Excel or any other worksheet files or SAS-XPT (as is done by a number of our customers).
In case you receive your captured data as SAS files, you can also use the free SAS Clinical Standards Toolkit" to convert your data to CDISC ODM.
OpenClinica® is a trademark of Akaza Research LLC in the United States
and other countries.
XML4Pharma is not a certified partner, reseller, or affiliate
of Akaza Research LLC, and is not certified, approved, or sponsored by
Akaza Research LLC in connection with the installation, operation,
maintenance or support of the OpenClinica® software platform, the
OpenClinica® Enterprise software platform or any other Akaza
products.
